Rooted in 30 years of stimulating all of life's moments
IMPLANT THE FUTURE OF SEIZURE CONTROL
Epilepsy (US) — The VNS Therapy System is indicated for use as an
adjunctive therapy in reducing the frequency of seizures
in patients 4 years of age and older with partial onset
seizures that are refractory to antiepileptic medications.
VNS TherapyTM has been used to deliver life-changing treatment to more than 175,000 people with drug-resistant epilepsy, including 50,000+ children2
VNS TherapyTM offers a unique neuromodulation mechanism of action3
VNS Therapy™ works through monoaminergic modulation of limbic and thalamocortical circuitry.
The unique mechanism of action involves the bottom-up modulation of seizure-propagating circuitry.
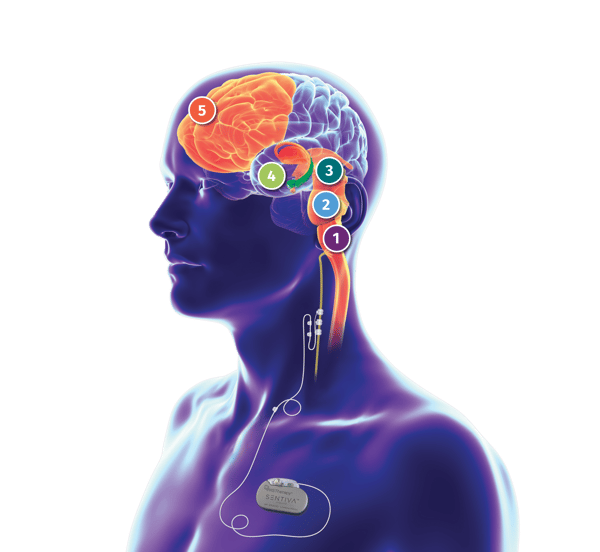
The vagal afferent network
Vagal afferents primarily project to the nucleus tractus solitarius (NTS), which then sends fibers to other brainstem nuclei that modulate subcortical and cortical activity. This vagal afferent network is likely the neural substrate of the anti-seizure effects of VNS Therapy.
.png?width=455&height=260&name=vns-man-key%20(1).png)
.png)
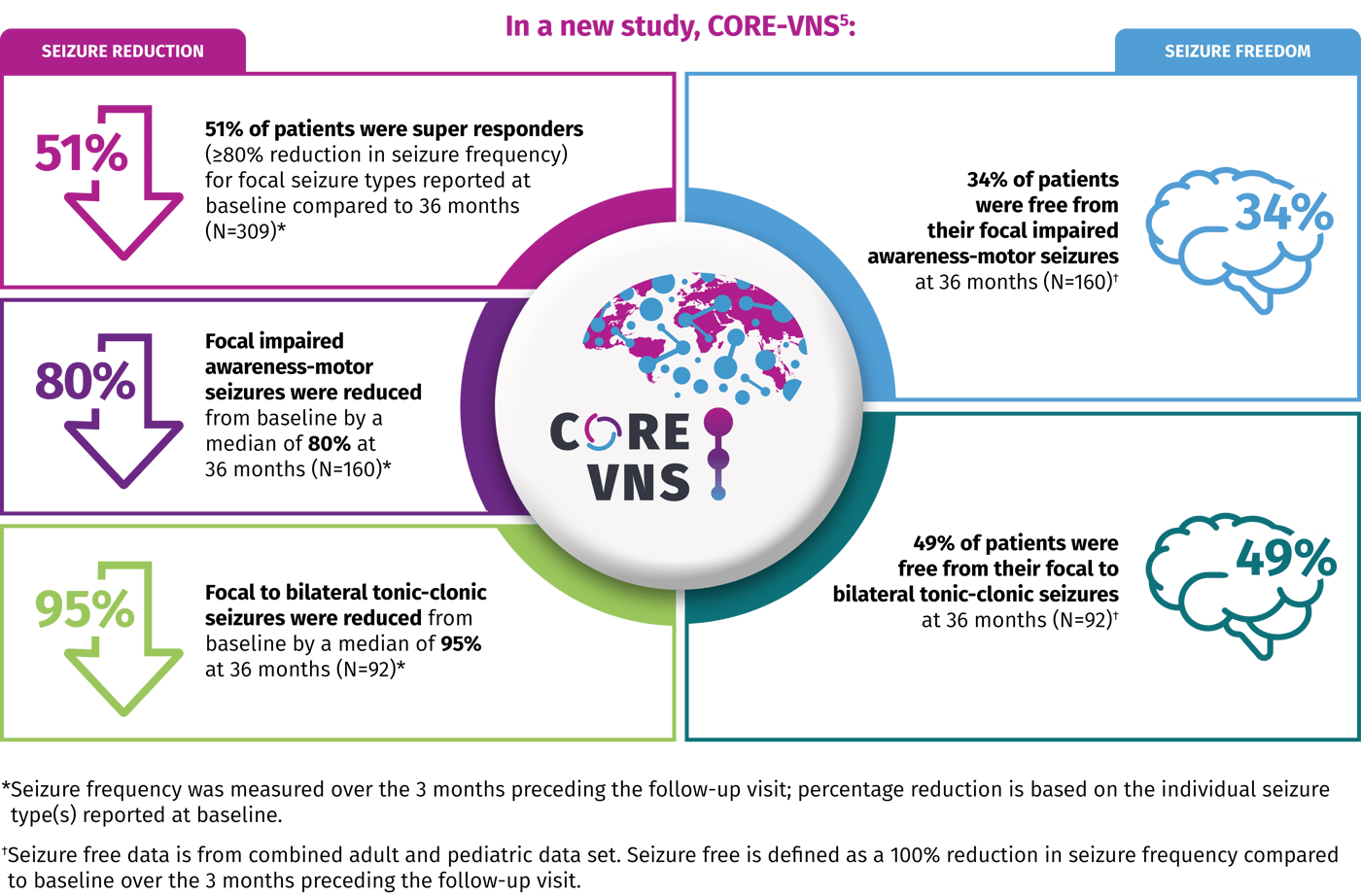
CORE-VNS is the Largest Global, Prospective, Real-world Study of VNS Therapy Outcomes in People with DRE4
The purpose of this study is to evaluate clinical outcomes and safety data in patients with Drug-Resistant Epilepsy (DRE) treated with VNS TherapyTM in an open-label, real-world setting and provide extensive, long-term data that can offer valuable insights. CORE-VNS analyzes individual seizure types according to the 2017 ILAE classification.
800+ Subjects

61 Sites

16 Countries

36 Months of Continuous Monitoring

VNS Therapy is approved as a treatment for partial-onset (focal) seizures. Lennox-Gastaut Syndrome (LGS) may include focal seizures. VNS Therapy is not indicated for the specific treatment of LGS.
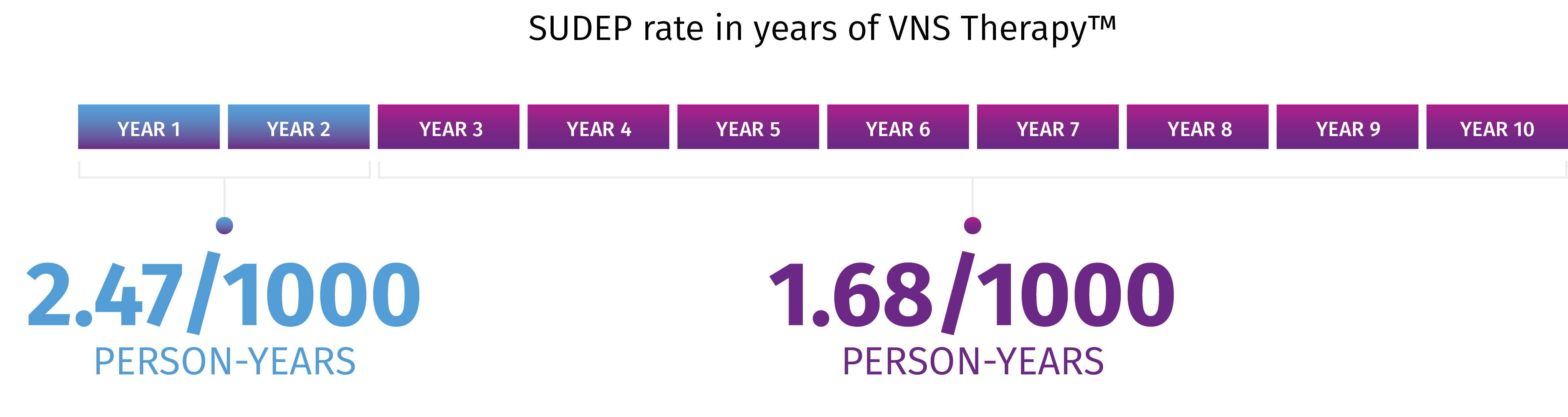
In a retrospective analysis, age-adjusted SUDEP rates decreased over time from years 1 to 2 (2.47/1000 person-years) to years 3 to 10 (1.68/1000 person-years).* A study on 277,661 person-years of up to 10 years of follow-up and 3689 deaths, including 632 cases of SUDEP, showed that long-term VNS Therapy is associated with lower SUDEP rates. The data suggest that SUDEP risk decreases during long-term follow-up of patients with refractory epilepsy receiving VNS Therapy.
*Limitations: This finding might reflect several factors, including ASM use, lack of a control group/seizure baseline, the natural long-term dynamic of SUDEP rate, attrition, and the impact of VNS Therapy. The role of each of these factors cannot be confirmed due to the limitations of study.
Treatment with VNS TherapyTM may be associated with lower sudden unexpected death in epilepsy (SUDEP) rates7
VNS TherapyTM can provide continued reduction in seizure frequency and improve the detrimental consequences of DRE
Compared to pharmacotherapy alone, adjunctive VNS Therapy is associated with a significantly decreased monthly risk of hospitalization and emergency department (ED) visits. Over a 24-month period (N=659, p <0.001)8:
All-cause ED visits decreased by 46%
Epilepsy-related ED visits decreased by 55%
All-cause hospitalizations and ED visits decreased by 42%
Most Common Side Effects of VNS Therapy9,10
VNS TherapyTM has no systemic or CNS side effects that are commonly associated with anti-seizure medications11
-
Can improve seizure control without contributing to ASM toxicity
-
No drug interactions — can be combined with any ASM regimen
In a prospective study over 3 years:
Across VNS Therapy studies, the most common side effects from stimulation include voice alteration/hoarseness, increased coughing, sore throat, prickling or tingling of the skin, and shortness of breath. Infection is the most common complication of the procedure.
In a survey of 200 VNS TherapyTM patients and caregivers12
INTENDED USE / INDICATIONS
Epilepsy (US) - The VNS Therapy System is indicated for use as an adjunctive therapy in reducing the frequency of seizures in patients 4 years of age and older with partial onset seizures that are refractory to antiepileptic medications.
References: 1. Elliot RE, Morsi A, Tanweer O, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10 years. Epilepsy Behav. 2011;20(3):478-483. 2. Data on file, LivaNova USA, Inc. Houston, TX. 2025. 3. Hachem LD, Wong SM, Ibrahim GM. The vagus afferent network: emerging role in translational connectomics. Neurosurg Focus. 2018;45(3):E2. 4. CORE-VNS. ClinicalTrials.gov Identifier: NCT03529045. Available from: https://clinicaltrials.gov/ct2/show/NCT03529045. 5. Data on file. CORE-VNS Clinical Study Report, LivaNova, USA, Inc. Houston, TX. June 2025. 6. Lyons P, Wheless J, Verner R, et al. Vagus nerve stimulation in Lennox-Gastaut syndrome: twenty-four-month data and experience from the CORE-VNS study. Epilepsia. 2025;00:1-10. 7. Ryvlin P, So EL, Gordon CM, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia. 2018;59(3):562-572. 8. Evans K, Stamas N, Li Q, et al. Impact of vagus nerve stimulation for the treatment of drug-resistant epilepsy on patterns of use and cost of health care services and pharmacotherapy: comparisons of the 24-month periods before and after implantation. Clin Ther. 2023;45(2):136-150. 9. VNS Therapy System Epilepsy Physician’s Manual (US), April 2025, LivaNova USA, Inc. Houston, TX. 10. Morris GL, et al. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53(7):1731-1735. 11. Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 2001;18(5):415-418. 12. Data on file, LivaNova USA, Inc., Houston, TX. 2021.